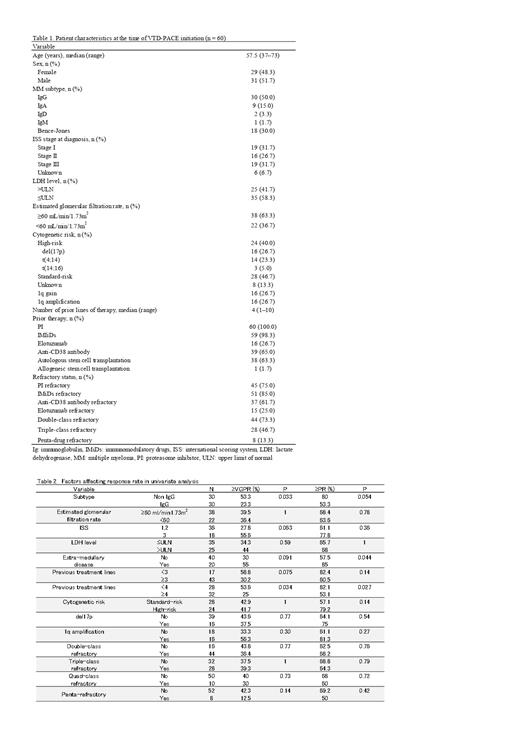
The treatment outcomes of multiple myeloma (MM) have drastically improved with the use of novel agents, including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and monoclonal antibodies. However, ultimately, the disease relapses and results in a fatal outcome. Even in cases of relapse, treatment outcomes have improved with the use of novel agents. However, in situations where novel agents have been exhausted or when the disease rapidly progresses, bortezomib (Velcade), thalidomide, dexamethasone, platinum (cisplatin), adriamycin (doxorubicin), cyclophosphamide, and etoposide (VTD-PACE) is frequently utilized, with reported effectiveness. However, its outcomes in the era of monoclonal antibodies remain unclear. Therefore, this retrospective cohort study assessed the clinical outcomes of 60 patients with RRMM (median four prior treatment lines) administered VTD-PACE at the Japanese Red Cross Medical Center between June 2016 and April 2023. The characteristics of the 60 patients are shown in Table 1. The VTD-PACE regimen consisted of 1 mg/m 2 of bortezomib on days 1, 4, 8, and 11 administered subcutaneously; 200 mg of thalidomide administered orally on days 1-4; 40 mg of dexamethasone administered orally on days 1-4; and a 4-day continuous intravenous infusion of 10 mg/m 2 cisplatin, 40 mg/m 2 cyclophosphamide, 40 mg/m 2 etoposide, and 10 mg/m 2 doxorubicin. The cisplatin dose was adjusted based on renal function, as determined by the treating physician. The responses were evaluated according to the International Myeloma Working Group response criteria. High-risk cytogenetic abnormalities (HRCA) included del (17p), t(4;14), and t(14;16). Double-class refractory disease was defined as refractoriness to PIs and IMiDs, whereas triple-class refractory disease was defined as refractoriness to PIs, IMIDs, and anti-CD38 monoclonal antibodies. Penta-drug refractory disease was defined as refractoriness to bortezomib, carfilzomib, lenalidomide, pomalidomide, and anti-CD38 antibodies. The median follow-up period was 11.1 months (range, 0.5-72.0 months), during which they received a median of two cycles of VTD-PACE. The overall response (ORR), stringent complete response, complete response, and very good partial response rates were 66.7% (40 patients), 13.3% (8 patients), 5% (3 patients), and 20% (12 patients), respectively. The results of univariate analysis for factors associated with response rate are shown in Table 2. The ORRs in patients with ≥4 and ≤3 prior lines were 53.1% and 82.1%, respectively ( P = 0.027). Extramedullary disease (EMD), refractoriness to anti-CD38 antibody, HRCA, and triple-class/penta-drug refractory disease did not significantly reduce the ORR. The median overall survival (OS) was 17 months with a median progression-free survival (PFS) of 9.8 months. The median PFS in patients with and without receiving hematopoietic stem cell transplantation (HSCT) or chimeric antigen receptor T-cell therapy (CART) following VTD-PACE were 9.8 and 5.1 months, respectively ( P = 0.023). The median OS in patients with and without renal dysfunction were 10.7 months and 21.5 months, respectively ( P = 0.0091). A trend for longer OS was observed in patients with HSCT or CART following VTD-PACE (P = 0.18). Therefore, VTD-PACE is useful as a bridging therapy for HSCT or CART, as a response is expected regardless of organ damage, disease risk, or history of anti-CD38 antibody use. However, the response rate decreased in late lines; therefore, its use in early lines is recommended.
Disclosures
Yogo:Janssen: Other: advisory fee; Takeda: Other: Remuneration for lecture . Kikuchi:Abbvie: Other: Remuneration for lecture; Janssen: Other: Remuneration for lecture ; Takeda Pharmaceutial Company Limited: Other: Remuneration for lecture ; Sanofi: Other: Remuneration for lecture; Bristol-Myers Squibb: Other: Remuneration for lecture. Tsukada:Sanofi S.A.: Other: Remuneration for lecture ; Takeda Pharmaceutial Company Limited: Other: Remuneration for lecture ; Janssen Pharmaceutical K.K.: Other: Remuneration for lecture. Suzuki:Abbvie GK: Other: Remuneration for lecture ; Takeda Pharmaceutical Company Limited: Other: Remuneration for lecture, Research Funding; Amgen: Other: Remuneration for lecture , Research Funding; Bristol Myers Squibb: Other: Remuneration for lecture, Research Funding; Novartis International AG: Other: Remuneration for lecture; ONO PHARMACEUTICAL CO., LTD.: Other: Remuneration for lecture; Sanofi S.A: Other: Remuneration for lecture; Janssen Pharmaceutical K.K.: Other: Remuneration for lecture. Ishida:Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Ono: Consultancy, Honoraria; Pfizer: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal